42 fda approved drug labels
Fda label requirements - jnrmyg.divadendesigns.shop FDA has described those differences in § 314.94(a)(8)(iv) (21 CFR 314.94(a)(8)(iv)) as including, for example, differences in formulation, bioavailability, or pharmacokinetics; labeling revisions made to comply with current FDA labeling guidelines or other guidance; or omission of an indication or other aspect of labeling protected by patent. Is It Really 'FDA Approved'? - U.S. Food and Drug Administration 10.05.2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ...
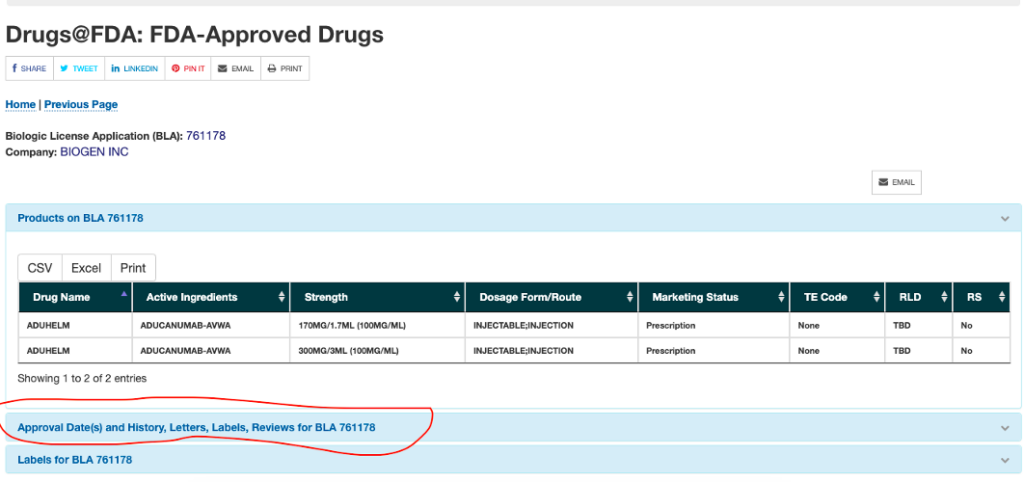
Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration Drug Name Active Ingredients Strength Dosage Form/Route Marketing Status TE Code RLD RS PARAGARD T 380A COPPER 309MG/COPPER INTRAUTERINE DEVICE;INTRAUTERINE Prescription None Yes Yes Labels for NDA...

Fda approved drug labels
FDA Antidepressant Drug Labels for Pregnant and Postpartum Women FDA Pregnancy Categories: Category C = Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of drug in pregnant women despite potential risks; Category D = There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing ... FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,... Drug Labeling Overview - Food and Drug Administration The openFDA drug product labels API returns data from these submissions for both prescription and over-the-counter (OTC) drugs. The labels are broken into sections, such as indications for use...
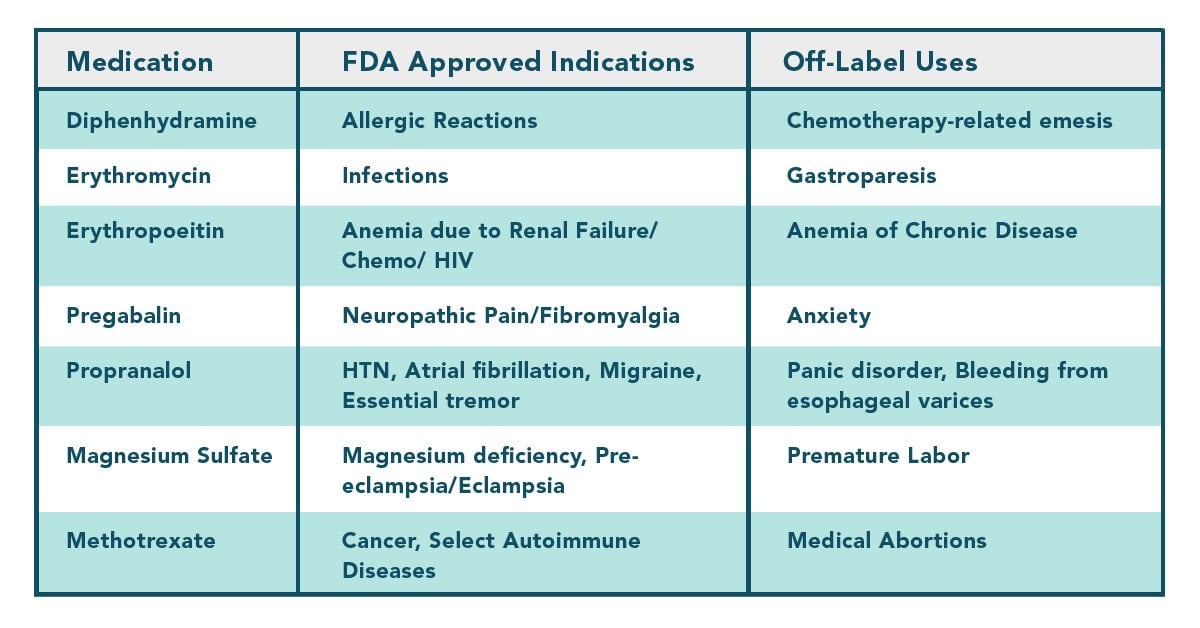
Fda approved drug labels. Fda label requirements - gcg.polskie-karmy.pl Finally, FDA regulations govern the precise cosmetic color and ingredient names that may be used on product labels . Thus, ... We apply our expertise and experience in FDA cosmetic labeling regulations and FTC cosmetic marketing regulations to ensure your cosmetics comply with federal requirements , while bearing useful claims for marketing. Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA 11.08.2022 · Pharmacogenomics can play an important role in identifying responders and non-responders to medications, avoiding adverse events, and optimizing drug dose. Drug labeling may contain information on ... Drug Approvals and Databases | FDA Drug and Biologic Approval and IND Activity Reports. Drug Trials Snapshots. Oncology (Cancer) / Hematologic Malignancies Approval Notifications. FDALabel. FDA Online Label Repository. FDA's ... Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 12/17/2018: SUPPL-54: Labeling-Medication Guide, Labeling-Package Insert
Sources of drug information: FDA-approved labeling and other ... - PubMed Abstract. To protect the public health and facilitate the safe and effective use of prescription drugs, the Food and Drug Administration (FDA) disseminates information through drug labeling, communication of safety issues, and the archiving of scientific reviews. The content and format requirements for professional labeling were revised in 2006 ... Animal Drugs @ FDA U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration ORIG-1. Approval. Type 2 - New Active Ingredient. STANDARD. Label is not available on this site. Action Date. Submission. Supplement Categories or Approval Type. Letters, Reviews, Labels, Patient Package Insert. Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 09/05/2013: SUPPL-33: Manufacturing (CMC) Label is not available on this site. 07/11/2001: SUPPL-16: Labeling Label is not available on this site. 06/22/1998: SUPPL-15: Manufacturing (CMC)-Control Label is not available on this site.
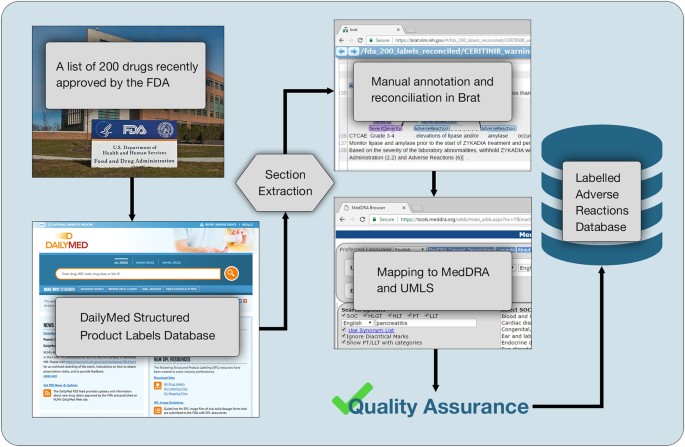
DailyMed DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical gases, devices, cosmetics, dietary supplements, and medical foods. The NLM provides DailyMed to the public and does not accept advertisements. Types of FDA Drug Labeling and Their Requirements - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. [3] An example of FDA-approved labeling is the Professional Package Insert (PPI). Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) Drug labels containing PGx information were obtained from Drugs@FDA and guidelines from PharmGKB were used to compare the actionability of PGx information in drug labels across therapeutic areas. The annual proportion of new drug approvals with PGx labeling has increased by nearly threefold from 10.3% (n = 3) in 2000 to 28.2% (n = 11) in 2020 ... FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor verified by FDA. The drug labeling on …
Drug Labels | FDA Drug Labels This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and...
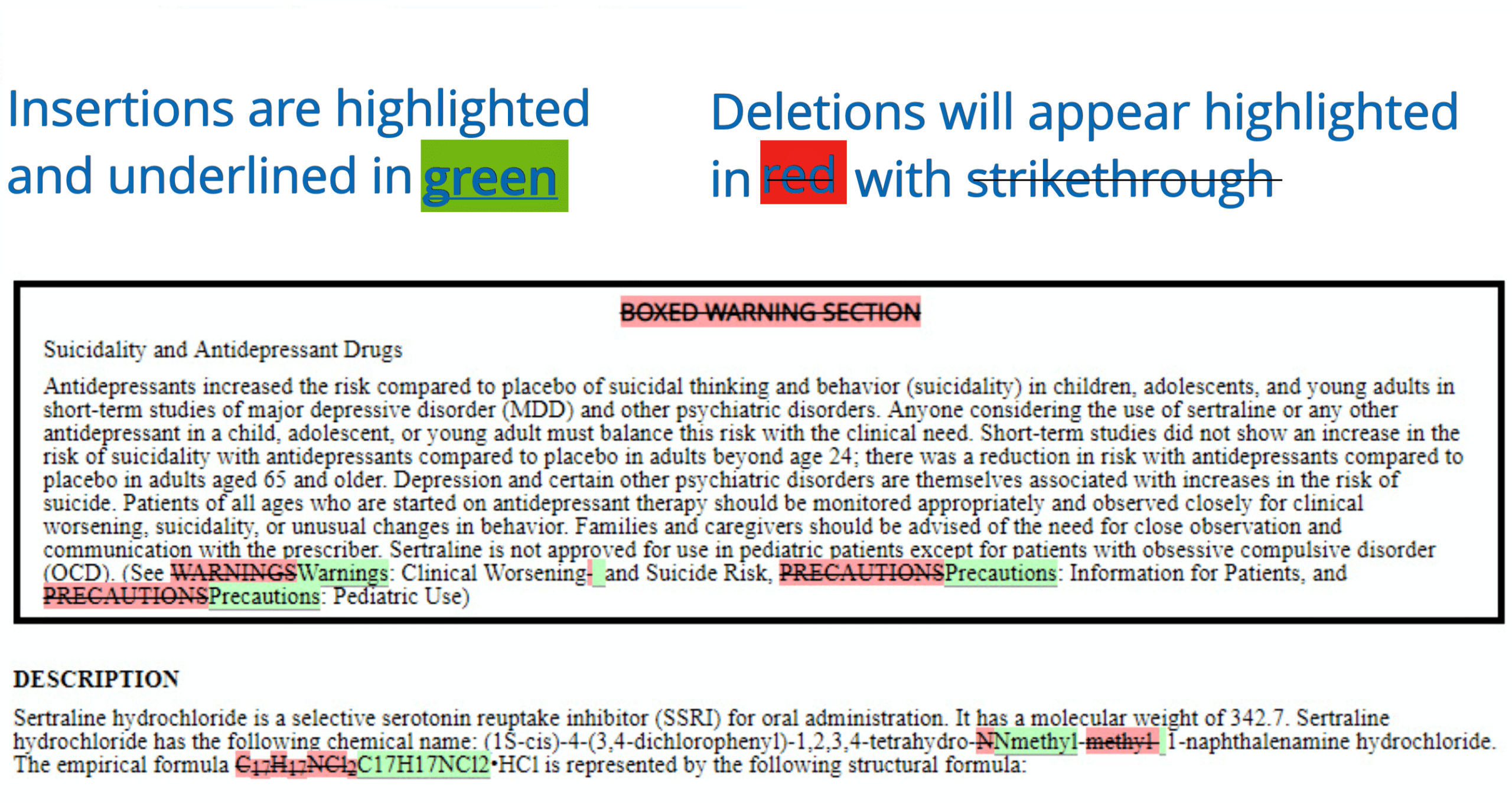
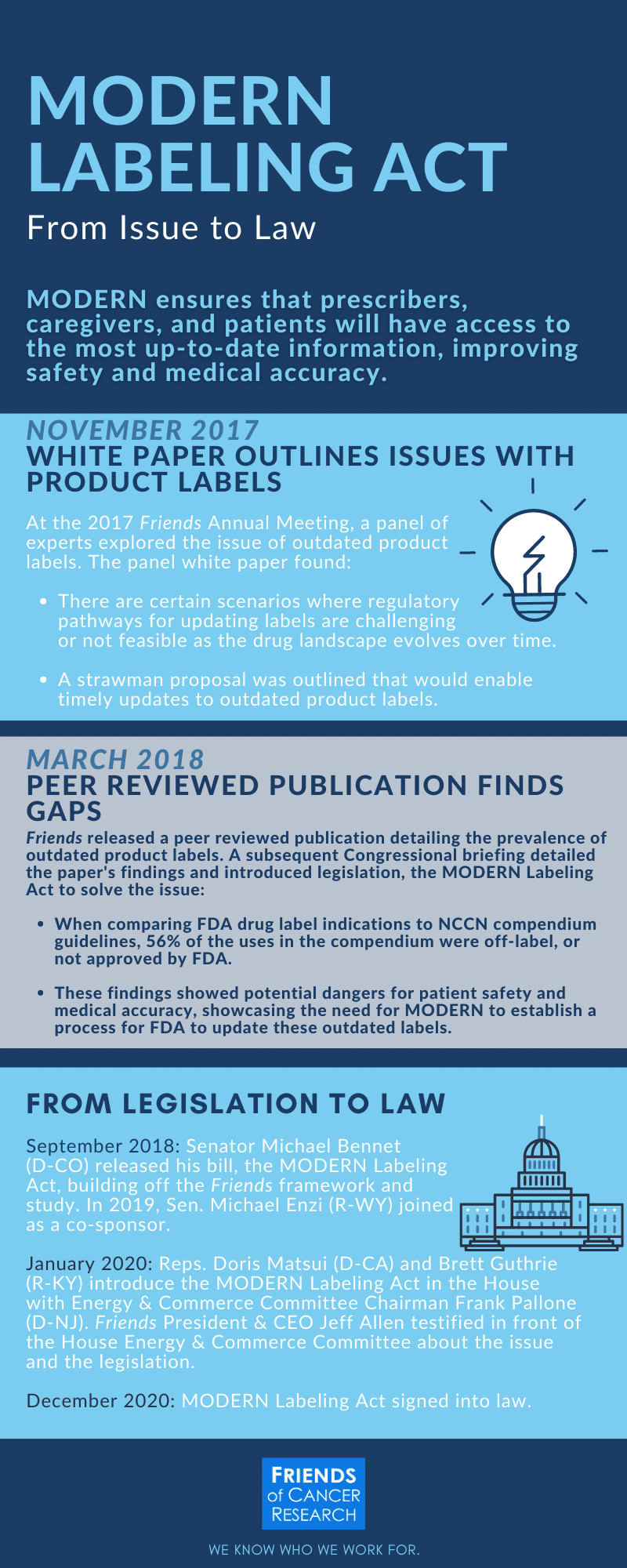
PDF label - Food and Drug Administration Initial U.S. Approval: 2004 -----RECENT MAJOR CHANGES----- Indications and Usage (1) 6/2012 Dosage and Administration (2.5) 6/2012 ... See 17 for PATIENT COUNSELING INFORMATION and FDA-approved Medication Guide Revised: 6/2012 . Reference ID: 3148643 ... Withdrawal of Antiepileptic Drugs (AEDs) 5.4. Suicidal Behavior and Ideation 5.5 ...
PDF Neurontin (gabapentin) Capsules Neurontin (gabapentin) Tablets ... FDA Approved Labeling Text dated 03/01/2011 Page 3 . Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing.
Drug Safety-related Labeling Changes (SrLC) Database Drug Safety-related Labeling Changes (SrLC) Database Overview: Updates to Safety Information in FDA-Approved Prescription Drug Labeling Contact FDA Toll Free (855) 543-3784, or (301) 796-3400...
FDA Approves Omlonti - drugs.com EMERYVILLE, Calif. & UBE, Japan--(BUSINESS WIRE) September 26, 2022 --Santen Inc., the U.S. subsidiary of Santen Pharmaceutical Co., Ltd. (Santen), and UBE Corporation (UBE) today announced that the U.S. Food and Drug Administration (FDA) has approved Omlonti (omidenepag isopropyl ophthalmic solution) 0.002% eye drops for the reduction of ...
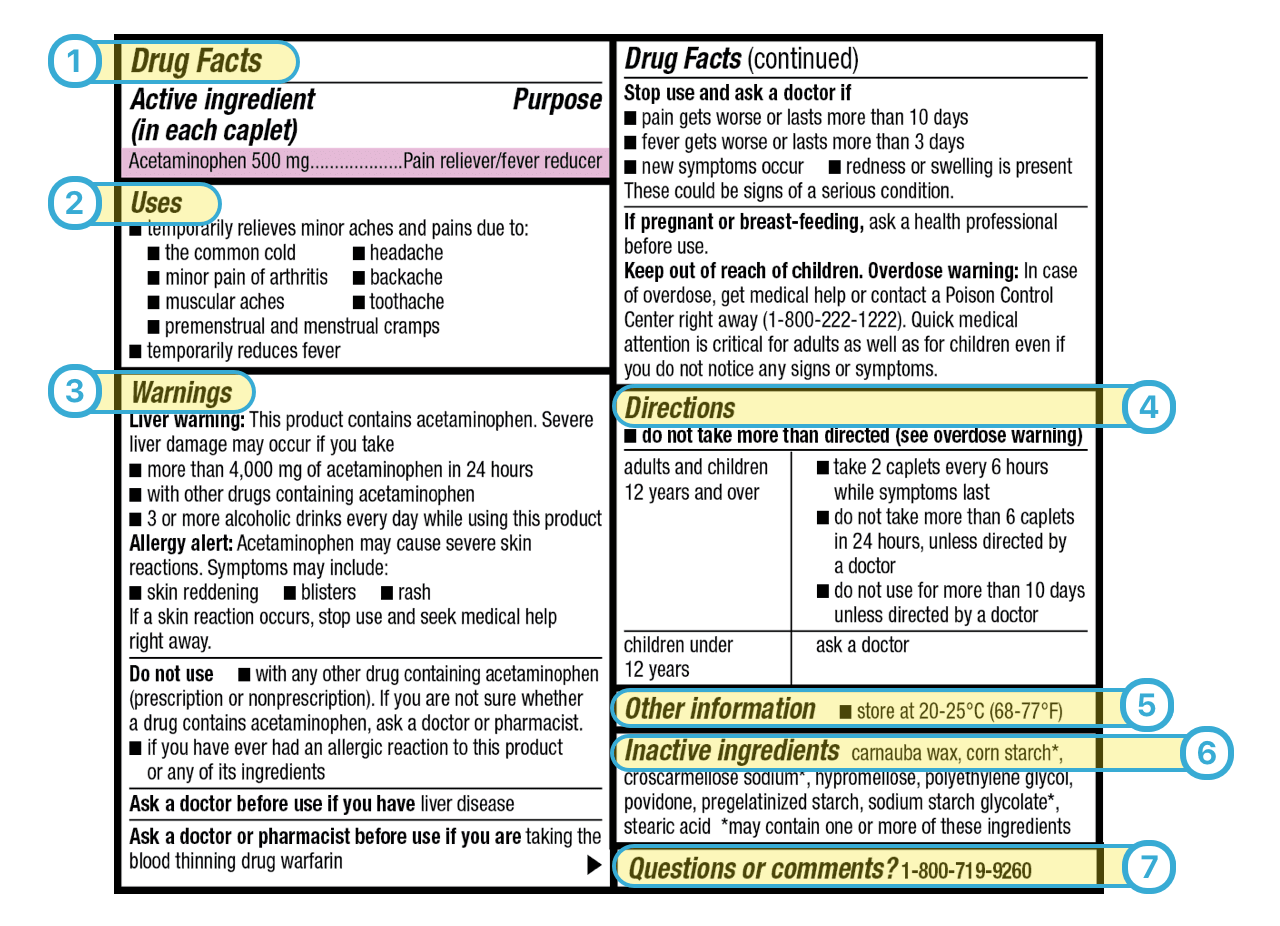
Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web...
Drug Labeling Overview - Food and Drug Administration The openFDA drug product labels API returns data from these submissions for both prescription and over-the-counter (OTC) drugs. The labels are broken into sections, such as indications for use...
FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...
FDA Antidepressant Drug Labels for Pregnant and Postpartum Women FDA Pregnancy Categories: Category C = Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of drug in pregnant women despite potential risks; Category D = There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing ...
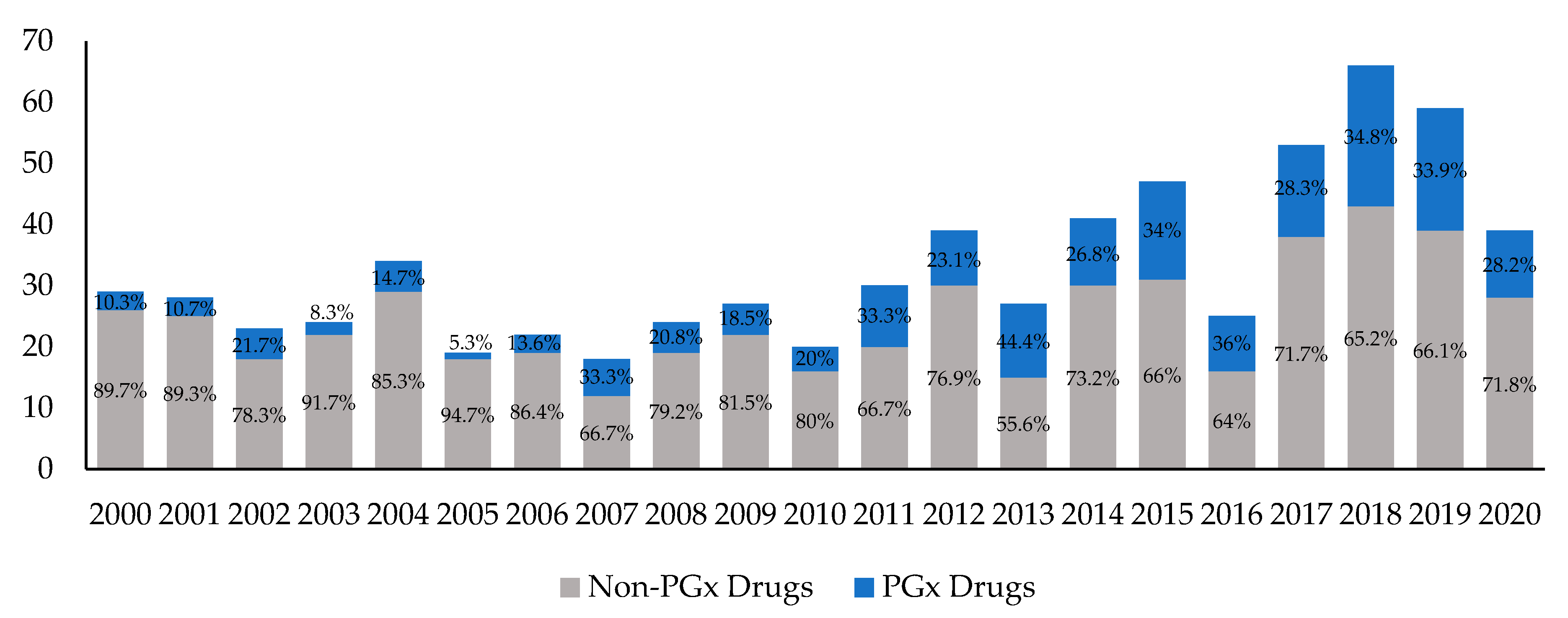

:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/4097058/flibanserin%20with%20DH%20v4%20(post-label)%20(1).jpg)




.jpg)





















Post a Comment for "42 fda approved drug labels"